CDM Training
What is CDM Training ?
Experienced professors and industry experts at GDS are providing online training in CDM course to make life science graduates and post graduates familiar about CDM and opportunities in the field.
Clinical Data Management (CDM) is the broad process which involves collection of data from clinical trials, further it involves integration, analytics and validation of collected data using the systematic CDM tools, SOPs and related softwares. CDM is a critical in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials and helps to produce a drastic reduction in time from drug development to marketing thus leading to a pronounced need for individuals and systems that provide CDM services.
Through our CDM Training Course, we make life science candidates aware with CDM processes beginning with the working knowledge and the course covers handling the digital technologies surrounding the collected data for integration, analytics and validation. Most importantly, the training focuses more on making candidates confident in the process of ensuring clinical data accuracy, validation for data completeness, ensure following SOPs, and consistency. All these aspects make freshers/researchers confident to attend interviews, attain great results in their on the job roles and responsibilities.
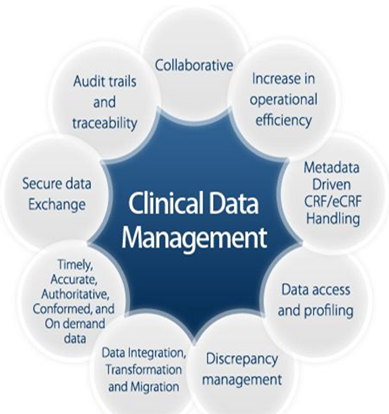
Job Role of CDM
The clinical data management process involves various steps and responsibilities to attain accurate data which supports the clinical trial results. This task further requires a multi-step process in ensuring integrity, consistency, and validity of every piece of appropriate data with accordance to drug approval regulatory agencies like the FDA and EMA.
Assured Assistance in Cracking Interviews
We help candidates by forwarding their profiles for all available vacancies in CDM through our partnered channels and we assure them assisting in cracking the interviews till they get a job. We extend our complete support 24/7.
Career Opportunities
Clinical Data Management (CDM) is an important department in pharmaceutical and contract research organizations that usually employs more people than any other department. Data Management teams develop tools for collection of data at clinical trial sites, quality check the collected data for errors and raise flags in case of any discrepancy identified. They develop tools for verification, validation and quality control of the data gathered during the clinical trial. Clinical data managers ensure the integrity and confidentiality of collected data whihc is maintained at all times. Various security controls are incorporated into the system to ensure that the data extracted from the clinical trials is secure and not prone to any open threats. Careers in Clinical Data Management are very promising. This field offers a wide scope of career options. Common positions available in Data Management departments are:
- • Clinical Data Manager
- • Bio-statistician
- • Clinical Data Coordinator
- • Clinical Data Entry Operator
- • Clinical Data Reviewer
- • Software Developers and Programmers with expertise in SAS, SPSS, Oracle Clinical, etc.
Thousands of companies offering jobs in CDM field with the salary package ranging from 3 Lakhs Per Annum to 3.5 Lakhs Per Annum. Most notable companies offering great employment opportunities are IQVIA, Novartis, TCS, Accenture and Parexel.
Who Can Join the Course ?
- • Graduates or Post Graduates in Life Science Courses
- • Bachelors or Masters in Medical Courses
- • Bachelors & Masters in Pharmacy
- • Bachelors & Masters in Computer Science
Course Details
Duration:
- • 30 Days - Regular Batch (5 Days a week, Monday to Friday, Daily 1 hr)
- • 10 Days - Weekend Batch (2 Days a week, Saturday and Sunday, 1.5 hr per Day)
Certification: Attendees will be awarded a certificate after successful completion of the course at the end.
Next Batch Starting on 1st October 2020 !!
Course Fees: Only Rs. 3000
For More details about the Course, Kindly contact through the below provided email:
cdmtraining@gervanora.com