We Offer You...
» Online support by email or telephone 24×7
» Assured confidentiality, safety and security as we operate through most trusted and reliable online payment systems
» Instant delivery of requested content in the desired form (PDF/PPT/Word Doc) to your provided email address after receiving the full payment.
» A Minimum Content Delivery initiation time of 9 hours
» An opportunity to be considered as an Expert Panel Member
» The provision of live, domain-specific updates

Autoimmune Diseases - Current & Forecasted Market Opportunities, Epidemiological Studies, Market Dynamics, Pipeline Analytics and r-NPV Analysis
DESCRIPTION:
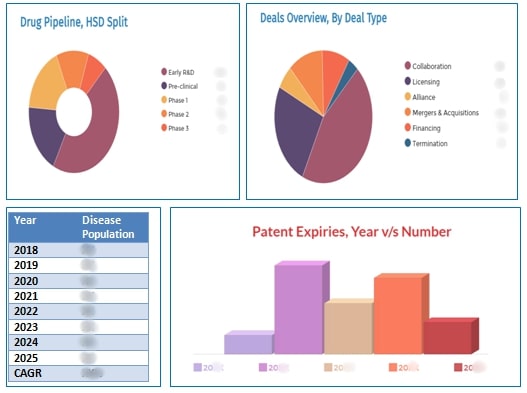
GervanoRA has assessed and analyzed the Autoimmune Diseases drugs pipeline by conducting a thorough secondary research and search of internal propriatory databases and has identified a number of pipeline molecules anticipated to enter the therapeutic market to serve the increasing disease population with unmet needs. We have classified the Autoimmune Diseases drugs pipeline into segments based on the molecules' Highest Stage of Development, Drug Class, Mechanism of Action, Route of Administration, Target and Company type. GervanoRA analysis predicts the Autoimmune Diseases drugs market size to increase in the near future with an increasing number of pipeline molecules entering clinical stages of development. Our comprehensive analysis on the Autoimmune Diseases drugs pipeline identified 1494 drug candidates undergoing different stages of development- from Early R&D to Pre-registration stage developing by around 1172 global companies actively. Among these, 205 drug candidates are in the Phase 3 stage of development, 379 candidates are in Phase 2 stage, 227 drug candidates are in Phase 1 stage of development and remaining molecules are in non-clinical stage of development. The drug pipeline consists of more number of preclinical drug candidates and followed by early stages of discovery. Along with these pipeline candicates, there are around 15 drug candidates in their pre-registration stage of development for different indications and geographies. GervanoRA has also analyzed that there are drug candidates who have been inactive or were discontinued due to various reasons from further developments. Below table shows total pipeline molecules for the treatment of autoimmune diseases gouped based on affected organ or body system.
An in-depth analysis based on the route of administration (ROA) of the therapeutic candidates sees that the major portion has been occupied by the oral and injectable candidates. The oral candidates occupying more than 50 percent in the drug pipeline are followed by injectable drug candidates making up a share of 41 percent. Inhalation and Topical drug candidates constitute a minor portion from the drug pipeline. Our analysis on geography has seen that, majority of the drug candidates targeting Autoimmune Diseases are anticipated to be filed for regulatory approvals in the US, followed by Europe. GervanoRA has performed pipeline analysis of Autoimmune Diseases molecules based on the target i.e. the type of Autoimmune Diseases receptor targeted. Autoimmune Disease area has witnessed a total of 291 major deals since 2010 to till the date. A year-by-year deals analytics have been provided highlighting the most active players in the competitive partnering landscape. An abrupt increase had been observed in the number of deals, increasing from 51 deals in the year 2018 to 94 deals in the year 2019. To add further, the year 2019 has marked some of the major deals in the healthcare industry, mainly the mergers and acquisition deals between Bristol-Myers Squibb Company and Celgene Corporation, and, Takeda Pharmaceutical Company Limited and Shire plc worth a total deal amount of $74 billion and $62 billion, respectively. As per the GervanoRA's analytics, a total of 43 mergers and acquisitions deals have occurred in the autoimmune disease area from the year 2015-2020 and 95 collaboration deals have taken place from the year 2010 till date. The Autoimmune Disease therapeutic area has witnessed a total of 65 major licensing deals from the year 2010 till date, from which 47 major licensing deals have occurred from the year 2017 till January 2020. There were a total of seventeen licensing deals in the year 2019, twelve in the year 2018 and fourteen in the year 2017. A total of 78 financing deals have occurred from the year 2010 till date, from which around 60 financing deals have been reported to take place in between 2017 and 2019.
As per GervanoRA analytics, Takeda Pharmaceutical's Entyvio IV will achieve China regulatory approvals in 2020 and Europe regulatory approval of Entyvio SC in 2021 for the treatment of Crohn's Diseases. Along with this molecule, Filgotinib of Gilead Sciences Inc/Galapagos NV, and PF-06410293 (Humira Biosimilar) of Pfizer may also get regulatory approvals in 2020. PF-06410293 (Humira Biosimilar) will get approval in Europe and Filgotinib in US for the treatment of Rheumatoid Arthritis. As per GervanoRA's estimated drug approval timelines, AbbVie's Risankizumab (Skyrizi) and Bristol-Myers Squibb's Ozanimod (RPC-1063) may achive regulatory approvals by 2022 in the US and by 2023 in Europe and Japan for the treatment of Crohn's Diseases. Along with these, there are other six pipeline molecules can compete to get US regulatory approvals in the year 2023 for the treatment of Crohn's Diseases. Dapagliflozin, Empagliflozin/BI10773, Ultra-Rapid Lispro/LY900014 and SAR341402 (Insulin Aspart) of AstraZeneca/Bristol-Myers Squibb, Boehringer Ingelheim International GmbH/Eli Lilly and Company, Eli Lilly and Company and Sanofi respectively are in pre-registration phase of development and may realize their regulatory approvals either end of 2020 or in 2021 for the treatment of Type 1 diabetes. Piclidenoson (CF101), Otilimab (Previously GSK3196165), Olokizumab, SM03 and TS-152 (Ozoralizumab) of Can-Fite BioPharma Ltd/Cipher Pharmaceuticals Inc/Gebro Pharma, GlaxoSmithKline Plc/MorphoSys AG, R Pharm, SinoMab BioScience and Taisho Pharmaceutical Holdings Inc/Ablynx are currently in Phase 3 stage of development with promising results and may get regulatory approvals in near future for the treatment of Rheumatoid Arthritis.
Some Of Our Clients Include: